In Japan, PLA has passed mold resistance tests and obtained hygiene and safety certification under Food Sanitation Act No. 370. Following the successful implementation of food utensil introduction trials in the cafeterias of the Ministry of Agriculture, Forestry and Fisheries (MAFF) and the Ministry of Economy, Trade and Industry (METI) (November 2003–March 2005, and March–April 2004), as well as the verification of its practicality and recyclability as the primary material for various food utensils at the Aichi Expo (2005 Japan International Exposition), PLA's future development in applications such as food utensils and foam sheets for fresh produce is highly anticipated.
Due to the asymmetric carbon atom in its structure, polylactic acid (PLA) exhibits optical activity. Currently, the most common experimental and industrial production method involves using L-monomers followed by ring-opening polymerization to prepare PLA. The specific material source, production process, application fields, and biodegradation cycle are illustrated in Figure 1.
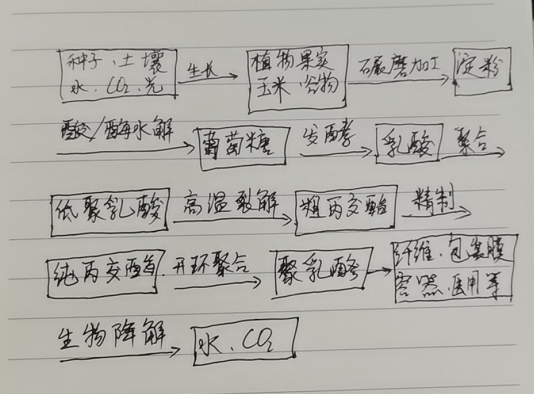 Figure 1: Biocycle Chain of PLA Green Plastic
Figure 1: Biocycle Chain of PLA Green Plastic
PLA is synthesized via chemical polymerization using L-lactic acid produced by starch fermentation. Thus, it is a polymer material that falls between microbially derived and chemically synthesized polymers. Amylase in corn, potatoes, and other starchy crops promotes hydrolysis to produce D-glucose, which is then converted to L-lactic acid through lactic acid fermentation. The L-lactic acid is subsequently subjected to thermal polycondensation to form low-molecular-weight lactic acid oligomers, which undergo ring-opening polymerization to yield poly-L-lactic acid (PLLA) with a molecular weight of 300,000–900,000. This method, known as the L-lactide process, is the most widely used. Recently, however, PLA with a molecular weight of 100,000–200,000 has also been successfully produced via the direct dehydration condensation of lactic acid (Mitsui Chemicals process).
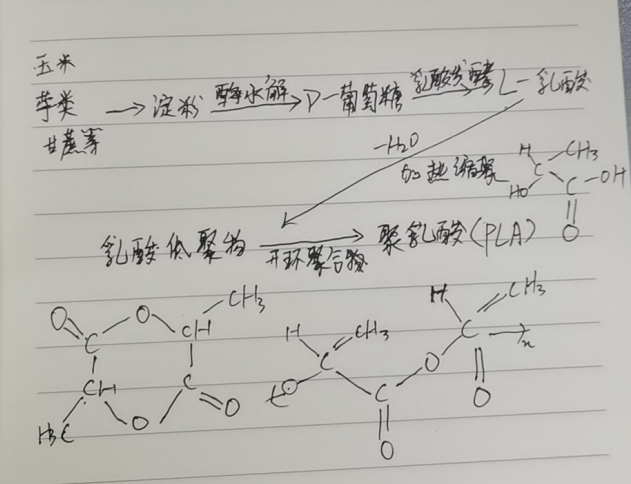 Figure 2: Synthesis of PLA via the L-lactide Process
Figure 2: Synthesis of PLA via the L-lactide Process
As shown in Figure 3, the theoretical yield of each stage in PLA synthesis from corn fermentation is presented. Based on this calculation, approximately 10 corn kernels can produce one A4-sized PLA film (weighing about 2g). With a global corn output of 550 million tons, utilizing a portion of waste corn for PLA production is sufficient to ensure adequate supply.
 Figure 3: Theoretical Yield of PLA Synthesized from Corn
Figure 3: Theoretical Yield of PLA Synthesized from Corn
The tensile strength and elongation at break of PLA films are nearly equivalent to those of polyethylene (PE). Taking biaxially oriented films as an example, PLA exhibits a tensile strength of 190 MPa and an elongation at break of 135%, compared to PE's 235 MPa and 130%, respectively. However, a key difference lies in their melting points: PE melts at 264°C, while PLA has a melting point of only 170°C.
Inferred from its chemical structure, the presence of an asymmetric carbon atom in the starting monomer results in two structural isomers: L-form and D-form, as illustrated in the figure. Previously, only L-lactic acid was thought to exist naturally, but microorganisms capable of synthesizing D-lactic acid have recently been discovered. When poly(L-lactic acid) (PLLA) synthesized from L-lactic acid and poly(D-lactic acid) (PDLA) synthesized from D-lactic acid are mixed at a 1:1 ratio in solution or molten state, their molecular chains align alternately in the crystal lattice to form a stereocomplex. In this stereocomplex, three turns of the right-handed helix and three turns of the left-handed helix are alternately oriented in the crystal, resulting in a melting point of 230°C—far exceeding that of the homopolymers (170°C). This overcomes the low heat resistance drawback of conventional polyesters, enabling PLA to be used as a high-strength, heat-resistant material.
Article Title: How is PLA in Biodegradable Bags Produced? URL: https://en.szxylp.com/technical-data/how-is-pla-in-biodegradable-bags-produced.html